 |
Matronics Email Lists
Web Forum Interface to the Matronics Email Lists
|
| View previous topic :: View next topic |
| Author |
Message |
klwerner(at)comcast.net
Guest
|
 Posted: Fri Apr 01, 2011 5:47 pm Post subject: Specific Gravity on Batteries Posted: Fri Apr 01, 2011 5:47 pm Post subject: Specific Gravity on Batteries |
 |
|
Dear E-Wizards,
I recently got a small YUASA Battery (15AH or so) and it came with 2
bottles of "GILL" branded electrolyte from a friend, as he was going
with a PC-680 in his bird.
The Yuasa is a wet cell battery with filler caps, and it has never
been filled, although it is about 15 years old. Package was never
opened until I got it, same with the electrolyte.
Why would I even bother with a battery like that, you may ask? A:
Because if is the perfect size to start my back up generator at the
hangar. So my question is this:
The battery came with papers that specified an automotive electrolyte
with a specific gravity of 1.285 (I believe).
However, the specific gravity of the Gill electrolyte says it is
1.265, so it is a bit different then the automotive stuff.
Can I use the included Gill electrolyte, or am I better off to just go
to NAPA and get the automotive electrolyte?
What is the main difference in operation between electrolytes with
different SG's? Different charging current?
Thanks all, Konrad
| | - The Matronics AeroElectric-List Email Forum - | | | Use the List Feature Navigator to browse the many List utilities available such as the Email Subscriptions page, Archive Search & Download, 7-Day Browse, Chat, FAQ, Photoshare, and much more:
http://www.matronics.com/Navigator?AeroElectric-List |
|
|
|
| Back to top |
|
 |
nuckolls.bob(at)aeroelect
Guest
|
 Posted: Fri Apr 01, 2011 8:57 pm Post subject: Specific Gravity on Batteries Posted: Fri Apr 01, 2011 8:57 pm Post subject: Specific Gravity on Batteries |
 |
|
At 09:43 PM 4/1/2011, you wrote:
| Quote: |
Dear E-Wizards,
I recently got a small YUASA Battery (15AH or so) and it came with 2
bottles of "GILL" branded electrolyte from a friend, as he was going
with a PC-680 in his bird.
The Yuasa is a wet cell battery with filler caps, and it has never
been filled, although it is about 15 years old. Package was never
opened until I got it, same with the electrolyte.
Why would I even bother with a battery like that, you may ask? A:
Because if is the perfect size to start my back up generator at the
hangar. So my question is this:
The battery came with papers that specified an automotive electrolyte
with a specific gravity of 1.285 (I believe).
However, the specific gravity of the Gill electrolyte says it is
1.265, so it is a bit different then the automotive stuff.
Can I use the included Gill electrolyte, or am I better off to just go
to NAPA and get the automotive electrolyte?
What is the main difference in operation between electrolytes with
different SG's? Different charging current?
|
It's not a critical application so the
risks are low. Assuming the battery is not subject
to other vagaries of age, then the .020 difference
in the electrolyte only accounts for perhaps
25% of capacity. If all you need it for is
getting the engine started, then I'd
go ahead with the acid you have. If there
are no moisture tight seals on the cells,
I suspect that there are larger questions
than S.G. of the electrolyte after 15 years.
I'd put the juice in and stick a battery maintainer
on it for a day or so and put it in service.
Bob . . .
| Quote: | Thanks all, Konrad
-----
No virus found in this message.
Checked by AVG - www.avg.com
|
Bob . . .
| | - The Matronics AeroElectric-List Email Forum - | | | Use the List Feature Navigator to browse the many List utilities available such as the Email Subscriptions page, Archive Search & Download, 7-Day Browse, Chat, FAQ, Photoshare, and much more:
http://www.matronics.com/Navigator?AeroElectric-List |
|
|
|
| Back to top |
|
 |
klwerner(at)comcast.net
Guest
|
 Posted: Fri Apr 01, 2011 9:23 pm Post subject: Specific Gravity on Batteries Posted: Fri Apr 01, 2011 9:23 pm Post subject: Specific Gravity on Batteries |
 |
|
Thank you Bob!!!
On Apr 1, 2011, at 9:47 PM, Robert L. Nuckolls, III wrote:
| Quote: |
>
At 09:43 PM 4/1/2011, you wrote:
>
> >
>
> Dear E-Wizards,
>
> I recently got a small YUASA Battery (15AH or so) and it came with 2
> bottles of "GILL" branded electrolyte from a friend, as he was going
> with a PC-680 in his bird.
>
> The Yuasa is a wet cell battery with filler caps, and it has never
> been filled, although it is about 15 years old. Package was never
> opened until I got it, same with the electrolyte.
>
> Why would I even bother with a battery like that, you may ask? A:
> Because if is the perfect size to start my back up generator at the
> hangar. So my question is this:
>
> The battery came with papers that specified an automotive electrolyte
> with a specific gravity of 1.285 (I believe).
>
> However, the specific gravity of the Gill electrolyte says it is
> 1.265, so it is a bit different then the automotive stuff.
>
> Can I use the included Gill electrolyte, or am I better off to just
> go
> to NAPA and get the automotive electrolyte?
>
> What is the main difference in operation between electrolytes with
> different SG's? Different charging current?
It's not a critical application so the
risks are low. Assuming the battery is not subject
to other vagaries of age, then the .020 difference
in the electrolyte only accounts for perhaps
25% of capacity. If all you need it for is
getting the engine started, then I'd
go ahead with the acid you have. If there
are no moisture tight seals on the cells,
I suspect that there are larger questions
than S.G. of the electrolyte after 15 years.
I'd put the juice in and stick a battery maintainer
on it for a day or so and put it in service.
Bob . . .
> Thanks all, Konrad
>
> -----
> No virus found in this message.
> Checked by AVG - www.avg.com
Bob . . .
|
| | - The Matronics AeroElectric-List Email Forum - | | | Use the List Feature Navigator to browse the many List utilities available such as the Email Subscriptions page, Archive Search & Download, 7-Day Browse, Chat, FAQ, Photoshare, and much more:
http://www.matronics.com/Navigator?AeroElectric-List |
|
|
|
| Back to top |
|
 |
nuckolls.bob(at)aeroelect
Guest
|
 Posted: Mon Apr 04, 2011 7:40 am Post subject: Specific Gravity on Batteries Posted: Mon Apr 04, 2011 7:40 am Post subject: Specific Gravity on Batteries |
 |
|
On second thought, *IF* I were to use this battery in an airplane
application instead of a generator, then what would you recommend?
Would you rather use the recommended automotive electrolyte ((at)1.285),
as a 25% dip in capacity/performance seems pretty steep by using the
provided GILL juice ((at)1.265).
I wasn't too clear on that answer. Consider this chart
taken from one of many tomes on batteries;
==========
Specific Gravity Readings � �True� State of Charge
The specific gravity (SG) of the battery acid or electrolyte is the truest and most absolute measure of a battery�s state of charge. The SG reading is NOT greatly or adversely affected by the load on the battery. Basically if a battery is 50% charged, it will read a specific gravity of 1.200 (see Table 1), regardless of whether the battery is on charge, being discharge or being stored. This is not the case for voltage readings.
Table 1. SG vs. Voltage
% Charged Specific Gravity
100% 1.255 � 1.275
75% 1.215 � 1.235
50% 1.180 � 1.200
25% 1.155 - 1.165
0% 1.110 - 1.130
====================================
Notice that here is a 'range' of values
that can represent state of charge for
any particular battery with a delta of
20 points out of 1300. Other books may
differ to some degree . . . for example:
===========
Specific gravity does, of course, vary with temperature and the quantity of electrolyte in a cell. When the electrolyte is near the low-level mark, the specific gravity is higher than nominal and drops as water is added to the cell to bring the electrolyte to the full level. The volume of electrolyte expands as temperature rises and contracts as temperature drops, therefore affecting the density or specific gravity reading. As the volume of electrolyte expands, the readings are lowered and, conversely, specific gravity increases with colder temperatures.
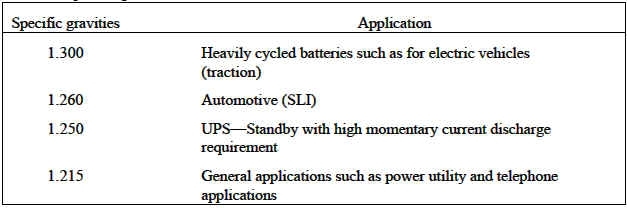
The specific gravity for a given battery is determined by the application it will be used in, taking into account operating temperature and battery life. Typical specific gravities for certain applications are shown in Table 1.
[img]cid:.0[/img][/i][/b]
The sum total of all information about batteries in general
will not help us much for your situation. We are
not privy to design goals and design characteristics of the
battery that was originally paired with the acid you have,
nor the acid that was paired with the battery you have.
My "25%" observation was based on a particular offering
of data from the 'net but it may not be relevant to your
particular acid/battery pair. The point I intended to
make was this. Given the low proposed, low criticality
application for this particular battery . . . combined
with now good knowledge of the battery's condition after
so long in storage, there was no compelling reason not
to use the acid you have with the battery you have.
The amount of GILL juice I got with this battery seems to be more then
enough to fill many batteries of this size, so instead of opening it,
why not simply get a smaller amount of automotive 1.285 instead?
It's your experiment. I have no basis to suggest that
one will be observably 'better' than the other . . . or
that the battery is even capable of delivering minimal
performance for the application proposed. Put the juice
in, top the critter off, and load/cap check it. If the
numbers are disappointing, deep cycle it a couple of times
and they MIGHT improve.
Besides difference in output, are there any differences in current and/ or voltage at which you would charge batteries with differing SG's? I do own a "BatteryMinder" model 12248, made by VDC Electronics which
allows various settings of battery type: Gel, Flooded, AGM (although I
do not know which voltages each setting represents), ...as well as
either a 2, 4 or 8 ampere charging current.
Consider that when you put any battery into service
the charging voltage is what the charging voltage
is. The charging current may be as much as the
rating for the alternator. The idea that one
can get 'better service' from any battery by
charging/maintaining it with a device having lots
of bells-and-whistles is not supported by a review
of in-service conditions.
Super-whippy battery chargers are much like Windows.
There are a few features that everybody uses that
cover their needs 95% of the time. All the rest is
'cool' . . . but . . .
Battery minders having a bulk-charge, top-off, and
terminated by a sustaining mode just above 13
volts is, for all practical purposes, good for
95% of our needs.
If you'd like to explore battery design and
performance issues in more detail, check out the
offerings by "Mr. Battery" hisself . . .
Mr. Isidor Buchmann who founded
http://www.cadex.com/
Talk about bells and whistles! Cadex products are
indeed finely tuned to specific battery technologies
used in demanding situation where costs of ownership
a fleet of battery users can be significantly influenced
by using the right tools and processes for battery
maintenance.
Isador has shared a great deal about the inner secrets
of batteries at:
http://batteryuniversity.com/
Having offered all this 'good stuff' on batteries, it's
time to "burst the bubble." When a really good battery
designer teams with a really good battery manufacturer
they'll tell you lots of good things they've learned about
how to get the most from their product. Their advice will
be based on hours of laboratory experiments. Then the
after-market folks will jump in with battery maintenance
accessories with lots of bells-and-whistles to compliment
those tests. But a big disconnect occurs when we take all
this data on deeply cycled batteries tested in laboratory
environments and put them on our garden tractors . . .
or airplanes.
The ideal engine cranking battery is NEVER deep cycled.
Once the engine taps 5% of capacity to get going, the
engine driven power source picks up the loads and replaces
the 5% taxation of capacity.
Then we depart from 'ideal' by walking away leaving the
master switch on . . . for two weeks. Our charging voltage
and recharge current values are NEVER in concert with
those laboratory ideas. Then, perhaps we suffer an
alternator out-event and for the first time since the
battery was installed. The pilot has to consider . . . .
Hmmmm . . . wonder what the CAPACITY of that battery
is? How does THAT condition stack up with things I'd
like to have working for then next few hours?
This is why I've suggested for years that all the smart
chargers in the world attached to the world's finest
battery products IS NOT a replacement for the owner/
operator KNOWING what the battery capacity is . . . when
and if that knowledge is important (poking holes in clouds
or long trips over unfriendly terrain at night).
I certainly do appreciate your ongoing input and expertise about
anything electric on the Matronics list!!!
My pleasure sir.
Bob . . .
| | - The Matronics AeroElectric-List Email Forum - | | | Use the List Feature Navigator to browse the many List utilities available such as the Email Subscriptions page, Archive Search & Download, 7-Day Browse, Chat, FAQ, Photoshare, and much more:
http://www.matronics.com/Navigator?AeroElectric-List |
|
| Description: |
|
| Filesize: |
58.6 KB |
| Viewed: |
3418 Time(s) |
|
|
|
| Back to top |
|
 |
|
|
You cannot post new topics in this forum
You cannot reply to topics in this forum
You cannot edit your posts in this forum
You cannot delete your posts in this forum
You cannot vote in polls in this forum
You cannot attach files in this forum
You can download files in this forum
|
Powered by phpBB © 2001, 2005 phpBB Group
|



