 |
Matronics Email Lists
Web Forum Interface to the Matronics Email Lists
|
| View previous topic :: View next topic |
| Author |
Message |
stuart(at)stuarthutchison
Guest
|
 Posted: Wed May 02, 2012 2:38 am Post subject: Aluminum bronze - conductivity Posted: Wed May 02, 2012 2:38 am Post subject: Aluminum bronze - conductivity |
 |
|
G'day Bob,
Do you reckon aluminum bronze is suitable to be used in place of brass for the main firewall ground ? It has plenty of copper in it, but are there any known nasties when it's used adjoining stainless steel - I'd like to turn a custom large-flanged firewall passthrough for the engine ground.
Kind regards, Stu<?xml:namespace prefix = o ns = "urn:schemas-microsoft-com:office:office" />
F1 Rocket VH-FLY http://www.mykitlog.com/RockFLY [url=about:www.teamrocketaircraft.com]www.teamrocketaircraft.com[/url]
[quote][b]
| | - The Matronics AeroElectric-List Email Forum - | | | Use the List Feature Navigator to browse the many List utilities available such as the Email Subscriptions page, Archive Search & Download, 7-Day Browse, Chat, FAQ, Photoshare, and much more:
http://www.matronics.com/Navigator?AeroElectric-List |
|
|
|
| Back to top |
|
 |
nuckolls.bob(at)aeroelect
Guest
|
 Posted: Wed May 02, 2012 6:19 am Post subject: Aluminum bronze - conductivity Posted: Wed May 02, 2012 6:19 am Post subject: Aluminum bronze - conductivity |
 |
|
At 05:33 AM 5/2/2012, you wrote:
| Quote: | G'day Bob,
Do you reckon aluminum bronze is suitable to be used in place of brass for the main firewall ground ? It has plenty of copper in it, but are there any known nasties when it's used adjoining stainless steel - I'd like to turn a custom large-flanged firewall passthrough for the engine ground.
|
Hmmmm . . . I have no reason to believe it's
any worse. Brass isn't the greatest of conductors
but the way we use it (large cross-sections and/or
short lengths) the electrical resistance doesn't
raise concerns. I don't know any specifics about its
reactivity with other metals but I think anything
against stainless is pretty low risk . . .
Perhaps others on the List have experience foundations
from which to advise you further . . .
Bob . . . [quote][b]
| | - The Matronics AeroElectric-List Email Forum - | | | Use the List Feature Navigator to browse the many List utilities available such as the Email Subscriptions page, Archive Search & Download, 7-Day Browse, Chat, FAQ, Photoshare, and much more:
http://www.matronics.com/Navigator?AeroElectric-List |
|
|
|
| Back to top |
|
 |
rparigoris
Joined: 24 Nov 2009
Posts: 796
|
 Posted: Wed May 02, 2012 6:59 am Post subject: Aluminum bronze - conductivity Posted: Wed May 02, 2012 6:59 am Post subject: Aluminum bronze - conductivity |
 |
|
Hi Stu
I compiled a number of links to galvanic and conductivity tables.:
http://www.europaowners.org/main.php?g2_itemId=87240
Click on "Download document", it's a word document with a number of links.
It kinda amazed me how poor a conductor brass and bronze is, but aluminium is very good and for all practical purposes as good as gold.
Stainless is a terrible conductor. Steel only slightly better. Guess what, lead and tin are very poor conductors! Did you ever see high amperage solder joints fail after time? It's because of the heating and cooling of the joint and fatigue failure of the solder BTW you can often rectify this from happening again by leaving a tail on the component, taking a solid strand of copper wire and wrapping the tail (and solder) and then soldering the both ends of this wire onto the board on the lands or pad to give triple the path for electrons to flow.
When you look at the galvanic tables, I forget if it was brass and stainless or aluminium and stainless, I think it was aluminium and stainless it is not a good choice. I forget if when I first made my 30,000,000 BTU Balloon Burner I used an aluminium restrictor for the pilot light inside stainless, or brass inside stainless. I think it was aluminium? Anyway it plugged up with white corrosion a few times and I lost the pilot light. After the second time I switched over to brass (of vise versa, you will be able to tell from the chart) I never had a problem again.
Good Luck.
Ron Parigoris
[quote][b]
| | - The Matronics AeroElectric-List Email Forum - | | | Use the List Feature Navigator to browse the many List utilities available such as the Email Subscriptions page, Archive Search & Download, 7-Day Browse, Chat, FAQ, Photoshare, and much more:
http://www.matronics.com/Navigator?AeroElectric-List |
|
|
|
| Back to top |
|
 |
henador_titzoff(at)yahoo.
Guest
|
 Posted: Wed May 02, 2012 7:11 am Post subject: Aluminum bronze - conductivity Posted: Wed May 02, 2012 7:11 am Post subject: Aluminum bronze - conductivity |
 |
|
With copper and aluminum, it's bound to be extremely low resistance, but the aluminum will corrode on the surface to form alumina, which may interfere with good connections. Why not send that puppy off to Eric at Perihelion Design to get it copper plated? Or better yet, why not go with copper bar? It's relatively cheap and known good material. Beats ripping out something that didn't work that well.
Henador Titzoff
From: "Robert L. Nuckolls, III" <nuckolls.bob(at)aeroelectric.com>
To: aeroelectric-list(at)matronics.com
Sent: Wednesday, May 2, 2012 10:18 AM
Subject: Re: Aluminum bronze - conductivity
At 05:33 AM 5/2/2012, you wrote:
| Quote: | G'day Bob,
Do you reckon aluminum bronze is suitable to be used in place of brass for the main firewall ground ? It has plenty of copper in it, but are there any known nasties when it's used adjoining stainless steel - I'd like to turn a custom large-flanged firewall passthrough for the engine ground.
|
Hmmmm . . . I have no reason to believe it's
any worse. Brass isn't the greatest of conductors
but the way we use it (large cross-sections and/or
short lengths) the electrical resistance doesn't
raise concerns. I don't know any specifics about its
reactivity with other metals but I think anything
against stainless is pretty low risk . . .
Perhaps others on the List have experience foundations
from which to advise you further . . .
Bob . . . [quote][b]
| | - The Matronics AeroElectric-List Email Forum - | | | Use the List Feature Navigator to browse the many List utilities available such as the Email Subscriptions page, Archive Search & Download, 7-Day Browse, Chat, FAQ, Photoshare, and much more:
http://www.matronics.com/Navigator?AeroElectric-List |
|
|
|
| Back to top |
|
 |
bbradburry(at)bellsouth.n
Guest
|
 Posted: Wed May 02, 2012 12:59 pm Post subject: Aluminum bronze - conductivity Posted: Wed May 02, 2012 12:59 pm Post subject: Aluminum bronze - conductivity |
 |
|
“When you look at the galvanic tables, I forget if it was brass and stainless or aluminium and stainless, I think it was aluminium and stainless it is not a good choice. I forget if when I first made my 30,000,000 BTU Balloon Burner I used an aluminium restrictor for the pilot light inside stainless, or brass inside stainless. I think it was aluminium? Anyway it plugged up with white corrosion a few times and I lost the pilot light. After the second time I switched over to brass (of vise versa, you will be able to tell from the chart) I never had a problem again.”
That’s pretty funny, Ron! :>) Clear, but funny!
B2
From: owner-aeroelectric-list-server(at)matronics.com [mailto:owner-aeroelectric-list-server(at)matronics.com] On Behalf Of rparigor(at)suffolk.lib.ny.us
Sent: Wednesday, May 02, 2012 10:58 AM
To: AeroelectricList
Subject: Re: AeroElectric-List: Aluminum bronze - conductivity
Hi Stu
I compiled a number of links to galvanic and conductivity tables.:
http://www.europaowners.org/main.php?g2_itemId=87240
Click on "Download document", it's a word document with a number of links.
It kinda amazed me how poor a conductor brass and bronze is, but aluminium is very good and for all practical purposes as good as gold.
Stainless is a terrible conductor. Steel only slightly better. Guess what, lead and tin are very poor conductors! Did you ever see high amperage solder joints fail after time? It's because of the heating and cooling of the joint and fatigue failure of the solder BTW you can often rectify this from happening again by leaving a tail on the component, taking a solid strand of copper wire and wrapping the tail (and solder) and then soldering the both ends of this wire onto the board on the lands or pad to give triple the path for electrons to flow.
When you look at the galvanic tables, I forget if it was brass and stainless or aluminium and stainless, I think it was aluminium and stainless it is not a good choice. I forget if when I first made my 30,000,000 BTU Balloon Burner I used an aluminium restrictor for the pilot light inside stainless, or brass inside stainless. I think it was aluminium? Anyway it plugged up with white corrosion a few times and I lost the pilot light. After the second time I switched over to brass (of vise versa, you will be able to tell from the chart) I never had a problem again.
Good Luck.
Ron Parigoris
| Quote: | | http://www.matronics.com/Navigator?AeroElectric-List |
0123456789
[quote][b]
| | - The Matronics AeroElectric-List Email Forum - | | | Use the List Feature Navigator to browse the many List utilities available such as the Email Subscriptions page, Archive Search & Download, 7-Day Browse, Chat, FAQ, Photoshare, and much more:
http://www.matronics.com/Navigator?AeroElectric-List |
|
|
|
| Back to top |
|
 |
rparigoris
Joined: 24 Nov 2009
Posts: 796
|
 Posted: Wed May 02, 2012 2:32 pm Post subject: Aluminum bronze - conductivity Posted: Wed May 02, 2012 2:32 pm Post subject: Aluminum bronze - conductivity |
 |
|
Hi B2
So you think having a power failure on your home built Hot Air balloon is funny? If you are not prepared they just like aeroplanes can in fact make a perfect 1 point landing! In other words balloons can and do in fact induce "P" factor", biological in nature (especially if you are over 50). No problem, I have that aspect pretty well "covered" if you know what I mean after you look at a few pictures  : :
http://www.europaowners.org/main.php?g2_itemId=28355
Ron Parigoris
Serious, I have a fully redundant pilot light that you can easily fly off of and deployable in seconds, and at least 3 other ignition sources that are a little more time consuming to use, but you can fly off of them as well. My two failures were pretty much non events.
When you look at the galvanic tables, I forget if it was brass and > stainless or aluminium and stainless, I think it was aluminium and > stainless > it is not a good choice. I forget if when I first made my 30,000,000 BTU > Balloon Burner I used an aluminium restrictor for the pilot light inside > stainless, or brass inside stainless. I think it was aluminium? Anyway it > plugged up with white corrosion a few times and I lost the pilot light. > After the second time I switched over to brass (of vise versa, you will be > able to tell from the chart) I never had a problem again." > > That's pretty funny, Ron! :>) Clear, but funny! > > B2 [quote][b]
| | - The Matronics AeroElectric-List Email Forum - | | | Use the List Feature Navigator to browse the many List utilities available such as the Email Subscriptions page, Archive Search & Download, 7-Day Browse, Chat, FAQ, Photoshare, and much more:
http://www.matronics.com/Navigator?AeroElectric-List |
|
|
|
| Back to top |
|
 |
stuart(at)stuarthutchison
Guest
|
 Posted: Thu May 03, 2012 12:08 am Post subject: Aluminum bronze - conductivity Posted: Thu May 03, 2012 12:08 am Post subject: Aluminum bronze - conductivity |
 |
|
Thanks guys.
I've made lots of aluminum and stainless parts for underwater photography and yes, the galvanic corrosion between them can be brutal immersed in an electrolyte liek sea water. Ultra Tef-Gel from B&C Specialty helps, but I suppose aluminum adjoining stainless is very common at the firewall and doesn't seem to present too many problems without an electrolyte ... I could be wrong, but that area stays pretty dry and I haven't witnessed much corrosion at the firewall on aircraft. I'm less worried about the aluminium bronze oxidising - a gas tight seal is obviously important because all the usual metal options oxidise, but there must be tens of thousands of aircraft with ground wires bonded direct to aluminium. I guess I'll just use di-electric grease to keep the air out at the point where the stud meets with the firewall, or perhaps make a phenolic bush to insulate it from the stainless, then bond the stud to a substantial part of the aluminum structure on the inside (such as an engine mounting bolt).
Kind regards, Stu<?xml:namespace prefix = o ns = "urn:schemas-microsoft-com:office:office" />
F1 Rocket VH-FLY http://www.mykitlog.com/RockFLY [url=about:www.teamrocketaircraft.com]www.teamrocketaircraft.com[/url]
From: owner-aeroelectric-list-server(at)matronics.com [mailto:owner-aeroelectric-list-server(at)matronics.com] On Behalf Of Henador Titzoff
Sent: Thursday, May 03, 2012 1:11 AM
To: aeroelectric-list(at)matronics.com
Subject: Re: AeroElectric-List: Aluminum bronze - conductivity
With copper and aluminum, it's bound to be extremely low resistance, but the aluminum will corrode on the surface to form alumina, which may interfere with good connections. Why not send that puppy off to Eric at Perihelion Design to get it copper plated? Or better yet, why not go with copper bar? It's relatively cheap and known good material. Beats ripping out something that didn't work that well.
Henador Titzoff
From: "Robert L. Nuckolls, III" <nuckolls.bob(at)aeroelectric.com>
To: aeroelectric-list(at)matronics.com
Sent: Wednesday, May 2, 2012 10:18 AM
Subject: Re: Aluminum bronze - conductivity
At 05:33 AM 5/2/2012, you wrote:
| Quote: | G'day Bob,
Do you reckon aluminum bronze is suitable to be used in place of brass for the main firewall ground ? It has plenty of copper in it, but are there any known nasties when it's used adjoining stainless steel - I'd like to turn a custom large-flanged firewall passthrough for the engine ground.
|
Hmmmm . . . I have no reason to believe it's
any worse. Brass isn't the greatest of conductors
but the way we use it (large cross-sections and/or
short lengths) the electrical resistance doesn't
raise concerns. I don't know any specifics about its
reactivity with other metals but I think anything
against stainless is pretty low risk . . .
Perhaps others on the List have experience foundations
from which to advise you further . . .
Bob . . . [quote]
ank" href="http://www.matronics.com/Navigator?AeroElectric-List">http://w=========
href="http://www.matronics.com/Navigator?AeroElectric-List">http://www.matronics.com/Navigator?AeroElectric-List
href="http://forums.matronics.com">http://forums.matronics.com
href="http://www.matronics.com/contribution">http://www.matronics.com/c
[b]
| | - The Matronics AeroElectric-List Email Forum - | | | Use the List Feature Navigator to browse the many List utilities available such as the Email Subscriptions page, Archive Search & Download, 7-Day Browse, Chat, FAQ, Photoshare, and much more:
http://www.matronics.com/Navigator?AeroElectric-List |
|
|
|
| Back to top |
|
 |
nuckolls.bob(at)aeroelect
Guest
|
 Posted: Thu May 03, 2012 5:28 am Post subject: Aluminum bronze - conductivity Posted: Thu May 03, 2012 5:28 am Post subject: Aluminum bronze - conductivity |
 |
|
At 03:06 AM 5/3/2012, you wrote:
Thanks guys.
I've made lots of aluminum and stainless parts for underwater photography and yes, the galvanic corrosion between them can be brutal immersed in an electrolyte liek sea water. Ultra Tef-Gel from B&C Specialty helps, but I suppose aluminum adjoining stainless is very common at the firewall and doesn't seem to present too many problems without an electrolyte ... I could be wrong, but that area stays pretty dry and I haven't witnessed much corrosion at the firewall on aircraft.
Agreed.
I'm less worried about the aluminium bronze oxidising - a gas tight seal is obviously important because all the usual metal options oxidise, but there must be tens of thousands of aircraft with ground wires bonded direct to aluminium.
The legacy process involves putting a tin-plated copper terminal down on a brightly buffed area of aluminum and applying lots of pressure with the attach hardware. The surface areas in gas-tight contact are, as you suggest, not subject to environmental stresses. The outside of the joint can get pretty cruddy with age without having the connection go bad.
I guess I'll just use di-electric grease to keep the air out at the point where the stud meets with the firewall, or perhaps make a phenolic bush to insulate it from the stainless, then bond the stud to a substantial part of the aluminum structure on the inside (such as an engine mounting bolt).
I think clean, grease and lots of pressure are key. Additional bonding is not useful.
Bob . . .
[quote][b]
| | - The Matronics AeroElectric-List Email Forum - | | | Use the List Feature Navigator to browse the many List utilities available such as the Email Subscriptions page, Archive Search & Download, 7-Day Browse, Chat, FAQ, Photoshare, and much more:
http://www.matronics.com/Navigator?AeroElectric-List |
|
|
|
| Back to top |
|
 |
Eric M. Jones

Joined: 10 Jan 2006
Posts: 565
Location: Massachusetts
|
 Posted: Thu May 03, 2012 7:16 am Post subject: Re: Short course in metals. Posted: Thu May 03, 2012 7:16 am Post subject: Re: Short course in metals. |
 |
|
| Quote: | | Why not send that puppy off to Eric at Perihelion Design to get it copper plated?...Henador |
The copper clad aluminum I sell is not plated. This wouldn't be good, plating always has pinholes and wears off. The Super-CCA I sell is "clad" which is a process where copper and aluminum are fused together. 10% of the diameter of the crossection is actually copper. Copperweld Inc., historically made lots of different stuff clad with copper by this process. It is important to know that there is a long history of aluminum wiring problems, but essentially NO HISTORY of CCA problems. The wire behaves very much like copper
Some notes of metals:
1) In many applications the conductivity of the metal is less important than the surface reactivity with atmospheric oxygen (or in the case of titanium only, with nitrogen). Impure aluminum oxide is the same as sapphire or corundum and is quite insulating. Copper oxide looks bad but still conducts electricity well. Same for silver. It can turn black and conduct well. Stainless steel, nickel, chrome and aluminum looks great initially but turns into an insulator. This usually happens slowly. Battery contacts made of stainless steel were once the bane of cheap electronics.
2) See: http://aerospacedefense.thomasnet.com/Asset/MIL-F-14072.pdf Finishes for Ground Based Electronic Equipment. There is probably an aircraft-version of this but it is all the same chemistry.
3) Gold has zero reactivity with the atmosphere. Gold is only a fairly- good conductor but is great for low voltage electrical contacts. Silver is the best conductor followed by copper, then aluminum.
4) Aluminum has over TWICE the conductivity per unit mass of any other metal. So learning how to use it can save weight. The electrical power industry uses far more aluminum than copper outside the home. Aluminum wiring in houses (that used electrical plugs and switches designed for copper) were retrofitted by adding a short pigtail of copper with special grease in a wirenut or crimp connector.
5) Galvanic corrosion depends on the Electrode potential in the electro-chemical series AND presence of an electrolyte--saltwater perhaps but water will do. If you put dissimilar metals together, but keep them air-tight and dry, there is no problem.
| | - The Matronics AeroElectric-List Email Forum - | | | Use the List Feature Navigator to browse the many List utilities available such as the Email Subscriptions page, Archive Search & Download, 7-Day Browse, Chat, FAQ, Photoshare, and much more:
http://www.matronics.com/Navigator?AeroElectric-List |
|
| Description: |
|
Download |
| Filename: |
Copper cables_Aluminum cables.pdf |
| Filesize: |
33.21 KB |
| Downloaded: |
575 Time(s) |
_________________
Eric M. Jones
www.PerihelionDesign.com
113 Brentwood Drive
Southbridge, MA 01550
(508) 764-2072
emjones(at)charter.net |
|
| Back to top |
|
 |
stuart(at)stuarthutchison
Guest
|
 Posted: Sat May 05, 2012 1:33 am Post subject: Aluminum bronze - conductivity Posted: Sat May 05, 2012 1:33 am Post subject: Aluminum bronze - conductivity |
 |
|
Hi Bob,
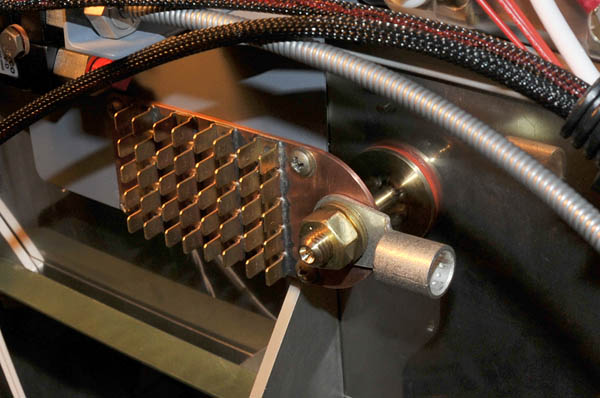
Maybe I didn't explain myself well, I'm a bit intrigued by your last comment. I appreciate that keeping the number of joins down is priceless, but since stainless is such a poor conductor, I would have expected to need to bond the stud to something else and not rely on current radiating out through the thin stainless firewall material? Probably overkill, but I made mine like the attached photo, which will have a short # 2 CCA fatwire between the forrest of ground tabs and an engine mounting bolt where there is chrome-moly structure bolted to a large part of the aluminium airframe. All of the bonding contact areas on my stud are at least 3/4", while the main shaft is 3/8 diameter, as are the threads and nuts. There is an silicone insulating washer on the front and back of the firewall for vibration absorbption as well.
.... I guess I'll just use di-electric grease to keep the air out at the point where the stud meets with the firewall, or perhaps make a phenolic bush to insulate it from the stainless, then bond the stud to a substantial part of the aluminum structure on the inside (such as an engine mounting bolt)" ...
"I think clean, grease and lots of pressure are key. Additional bonding is not useful."
| | - The Matronics AeroElectric-List Email Forum - | | | Use the List Feature Navigator to browse the many List utilities available such as the Email Subscriptions page, Archive Search & Download, 7-Day Browse, Chat, FAQ, Photoshare, and much more:
http://www.matronics.com/Navigator?AeroElectric-List |
|
| Description: |
|
| Filesize: |
64.26 KB |
| Viewed: |
8316 Time(s) |

|
| Description: |
|
| Filesize: |
82.64 KB |
| Viewed: |
8316 Time(s) |
|
| Description: |
|
| Filesize: |
53.9 KB |
| Viewed: |
8316 Time(s) |

|
|
|
| Back to top |
|
 |
henador_titzoff(at)yahoo.
Guest
|
 Posted: Sun May 06, 2012 3:50 pm Post subject: Aluminum bronze - conductivity Posted: Sun May 06, 2012 3:50 pm Post subject: Aluminum bronze - conductivity |
 |
|
Eric,
Thank you for correcting me about copper plating vs. copper clad and also for the additional information after that. I continuously run into people, even engineers, who think gold is the metal with best conductance. As you point out, gold has almost zero reactivity with the atmosphere, which makes it ideal for connector plating (or cladding!). It is also ideal inside ICs for thin plating and very thin wires, because it is so ductile.
I'd like to point out something, though. Below you say that silver is the best conductor, followed by copper then aluminum. Gold actually follows copper and then aluminum. What's really surprising is that calcium follows aluminum. The reasons calcium is not widely used as a conductor are reactance to the atmosphere, easy dissolution in water, and insufficient mechanical strength.
Henador Titzoff
From: Eric M. Jones <emjones(at)charter.net>
To: aeroelectric-list(at)matronics.com
Sent: Thursday, May 3, 2012 11:17 AM
Subject: Re: Aluminum bronze - conductivity
--> AeroElectric-List message posted by: "Eric M. Jones" <emjones(at)charter.net (emjones(at)charter.net)>
| Quote: | Why not send that puppy off to Eric at Perihelion Design to get it copper plated?...Henador
|
The copper clad aluminum I sell is not plated. This wouldn't be good, plating always has pinholes and wears off. The Super-CCA I sell is "clad" which is a process where copper and aluminum are fused together. 10% of the diameter of the crossection is actually copper. Copperweld Inc., historically made lots of different stuff clad with copper by this process. It is important to know that there is a long history of aluminum wiring problems, but essentially NO HISTORY of CCA problems. The wire behaves very much like copper
Some notes of metals:
1) In many applications the conductivity of the metal is less important than the surface reactivity with atmospheric oxygen (or in the case of titanium only, with nitrogen). Impure aluminum oxide is the same as sapphire or corundum and is quite insulating. Copper oxide looks bad but still conducts electricity well. Same for silver. It can turn black and conduct well. Stainless steel, nickel, chrome and aluminum looks great initially but turns into an insulator. This usually happens slowly. Battery contacts made of stainless steel were once the bane of cheap electronics.
2) See: http://aerospacedefense.thomasnet.com/Asset/MIL-F-14072.pdf Finishes for Ground Based Electronic Equipment. There is probably an aircraft-version of this but it is all the same chemistry.
3) Gold has zero reactivity with the atmosphere. Gold is only a fairly- good conductor but is great for low voltage electrical contacts. Silver is the best conductor followed by copper, then aluminum.
4) Aluminum has over TWICE the conductivity per unit mass of any other metal. So learning how to use it can save weight. The electrical power industry uses far more aluminum than copper outside the home. Aluminum wiring in houses (that used electrical plugs and switches designed for copper) were retrofitted by adding a short pigtail of copper with special grease in a wirenut or crimp connector.
5) Galvanic corrosion depends on the Electrode potential in the electro-chemical series AND presence of an electrolyte--saltwater perhaps but water will do. If you put dissimilar metals together, but keep them air-tight and dry, there is no problem.
--------
Eric M. Jones
www.PerihelionDesign.com
113 Brentwood Drive
Southbridge, MA 01550
(508) 764-2072
emjones(at)charter.net
Read this topic online here:
http://forums.matronics.com/viewtopic.php?p=372337#372337
Attachments:
http://forums.matronics.com//files/c//www.matronics.com/Navigator?AeroElectric-List" target="_blank">http://wnbsp; =
[quote][b]
| | - The Matronics AeroElectric-List Email Forum - | | | Use the List Feature Navigator to browse the many List utilities available such as the Email Subscriptions page, Archive Search & Download, 7-Day Browse, Chat, FAQ, Photoshare, and much more:
http://www.matronics.com/Navigator?AeroElectric-List |
|
|
|
| Back to top |
|
 |
Eric M. Jones

Joined: 10 Jan 2006
Posts: 565
Location: Massachusetts
|
 Posted: Sun May 06, 2012 4:32 pm Post subject: Re: Aluminum bronze - conductivity Posted: Sun May 06, 2012 4:32 pm Post subject: Re: Aluminum bronze - conductivity |
 |
|
Henador,
Thanks. Right you are.
For elements that can be drawn into wires--Bulk Conductivity: Silver, Copper, Gold, Aluminium. And Mass Conductivity: Aluminum, Copper, Silver, Zinc, Gold.
Eric
| | - The Matronics AeroElectric-List Email Forum - | | | Use the List Feature Navigator to browse the many List utilities available such as the Email Subscriptions page, Archive Search & Download, 7-Day Browse, Chat, FAQ, Photoshare, and much more:
http://www.matronics.com/Navigator?AeroElectric-List |
|
_________________
Eric M. Jones
www.PerihelionDesign.com
113 Brentwood Drive
Southbridge, MA 01550
(508) 764-2072
emjones(at)charter.net |
|
| Back to top |
|
 |
|
|
You cannot post new topics in this forum
You cannot reply to topics in this forum
You cannot edit your posts in this forum
You cannot delete your posts in this forum
You cannot vote in polls in this forum
You cannot attach files in this forum
You can download files in this forum
|
Powered by phpBB © 2001, 2005 phpBB Group
|





