 |
Matronics Email Lists
Web Forum Interface to the Matronics Email Lists
|
| View previous topic :: View next topic |
| Author |
Message |
nuckolls.bob(at)aeroelect
Guest
|
 Posted: Wed Jan 31, 2024 7:33 pm Post subject: One for the battery gurus: recovery from deep discharge Posted: Wed Jan 31, 2024 7:33 pm Post subject: One for the battery gurus: recovery from deep discharge |
 |
|
At 04:09 PM 1/30/2024, you wrote:
| Quote: | --> AeroElectric-List message posted by: Alec Myers <alec(at)alecmyers.com>
I have an SBS-J16 battery in an aircraft in which the battery master was left on for two weeks (I know).
When found, the open circuit terminal voltage had dropped to 2.2 volts. A Dewalt sophisticated battery charger didn’t want to have anything to do with it, |
Yeah . . . many smart chargers do a pre-assessment of the target
battery and will not take on the task unless the terminal voltage
is above some minimum level . . . I have a couple chargers that
do this.
A temporary parallel connection of the smart-charger and some
other voltage source, like another battery will often convince
the charger that it's time to go to work.
Now, recall the days long before RG/GlasMat batteries.
The 'wet' stuff inside was (and still is) a dilute mixture
of water and sulfuric acid. Pure water is a very poor
conductor of electrons . . . but adding some
combination of free ions like salt, sodium bicarbonate,
or sulfuric acid to the water and it becomes a ready
conductor of current.
Recall that we could test the relative state of
charge for a lead-acid battery by measuring the
electrolyte's DENSITY with a hydrometer. The
legacy float/in/glass hydrometer is generally
calibrated in density vs. state of charge
where electrolyte 12% greater than 1.000
(pure water) is zero-percent; 26% is full
charge.
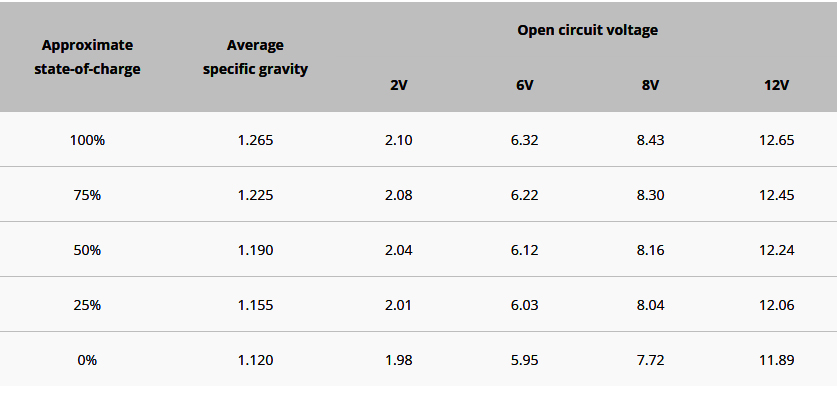
Note in attached figure (shamelessly stollen
off BatteryUniversity.com), 0% state of charge
on a 12 volt produces an open circuit reading
on the order of 11.9 volts.
You cited an open circuit voltage of 2.2 volts . . .
Hmmmm . . . less than 0% state of charge?
Actually, yes. Active material in the plates
had sucked still more acid from the electrolyte
than what would produce any useful energy
from the chemistry.
The closer to pure water . . . the more depressed
conductivity. Hence, first attempts to push energy
back into the battery will be met with lots of
resistance . . . no pun intended.
I recall reading a qualification test on
a Concorde battery document where a fully
discharged battery is dead-shorted for a
period of time after which a recharge
protocol calls for applying a higher than
normal voltage until significant recharge
current is observed. The test proceeds with
a normal constant voltage/constant current
charge. After top-off, the battery is cap-checked
and must demonstrate some minimum.
I dug around in the library but could not
come up with that document so I cannot quote
exact times and values. But note that this
is a quality test for a new battery. While
a certain level of degradation is expected,
the battery is EXPECTED to recover by some
minimum amount required for return to
service.
| Quote: | | so I’ve now put it on charge with my bench power supply at 14.4V limited to 4 amps. Initially the battery resistance was very high, and increasing - the voltage was limited at 14.4V and the current dropped from 1.3 amps, to about 1.1 amps, within a couple of minutes, and then started to ramp up, about 1mA per second. After being on charge for an hour or so, the current it is accepting has risen so the current limiting has kicked in, presently at 4 amps and the terminal voltage has dropped to 14.3 V. |
Yup, this is expected and you may well
recover this battery to some level for
continued service. After a 24-hour float
at 14.4, let it see idle for 24-hours
then do a cap check followed by recharge
and a load test.
| Quote: | | I”m curious why the resistance was so high to start with, why it has now dropped (to what seems ’normal’ charging behaviour to me) and also to know if this battery has a chance of resurrection,. It’s only a couple of years old, so if it will soldier on after its mistreatment, I would be happy. What are my chances, do you think? |
As Lord Kelvin oft admonished, if you
don't know the numbers, what you DO know
is of limited value.
Bob . . .
� ////
� (o o)
===========o00o=(_)=o00o=========
< Go ahead, make my day . . . >
< show me where I'm wrong. >
=================================
In the interest of creative evolution
of the-best-we-know-how-to-do based
on physics and good practice.
| | - The Matronics AeroElectric-List Email Forum - | | | Use the List Feature Navigator to browse the many List utilities available such as the Email Subscriptions page, Archive Search & Download, 7-Day Browse, Chat, FAQ, Photoshare, and much more:
http://www.matronics.com/Navigator?AeroElectric-List |
|
| Description: |
|
| Filesize: |
112.24 KB |
| Viewed: |
3587 Time(s) |
|
|
|
| Back to top |
|
 |
nuckolls.bob(at)aeroelect
Guest
|
 Posted: Sun Feb 04, 2024 12:32 pm Post subject: One for the battery gurus: recovery from deep discharge Posted: Sun Feb 04, 2024 12:32 pm Post subject: One for the battery gurus: recovery from deep discharge |
 |
|
At 11:25 AM 2/3/2024, you wrote:
| Quote: | In my case I had a newish deep cycle� 12v100a/h battery in an inverter drop to 3.6v...dont ask...
Anyway...the Victron charger didnt see the battery...put a 24v CTEK intelligent charger on it got it to 15v in about 30min. Changed over to the 7amp Victron 12v smart charger...
I had just about given up after about 5 days when suddenly the charge light went green!
Its been about a month and the battery seems fine. Maybe a bit of luck? |
It may have been quite predictable. When
the battery is discharge to LESS than zero,
i.e. open terminal volts less than 12.0
V and SG below 1.12, then what's left of
the acid is free do mischief on the battery's
innards . . . like sulfation at an
accelerated rate.
In the fully charged state, the negative plate
consists of lead; the positive plate is lead dioxide.
The electrolyte has a higher concentration acid
which is where chemical energy is stored.
When discharged, both the positive and negative plates
become lead sulfate; the electrolyte becomes less
acid and more water.
The lead sulfate is initially fine grains and
convertible back into hydrogen sulfide thus
increasing strength of the acid. Depending
on depth and duration of discharge, SOME of
the lead sulfate forms hard, insoluble crystals
thus diminishing battery CAPACITY.
Without a doubt, your battery has experienced
and extra-ordinary, deep-discharge which will
have consumed more of its service life than
if it had been subjected to a normal
discharge-recharge cycle.
Bob . . .
� ////
� (o o)
===========o00o=(_)=o00o=========
< Go ahead, make my day . . . >
< show me where I'm wrong. >
=================================
In the interest of creative evolution
of the-best-we-know-how-to-do based
on physics and good practice.
| | - The Matronics AeroElectric-List Email Forum - | | | Use the List Feature Navigator to browse the many List utilities available such as the Email Subscriptions page, Archive Search & Download, 7-Day Browse, Chat, FAQ, Photoshare, and much more:
http://www.matronics.com/Navigator?AeroElectric-List |
|
|
|
| Back to top |
|
 |
|
|
You cannot post new topics in this forum
You cannot reply to topics in this forum
You cannot edit your posts in this forum
You cannot delete your posts in this forum
You cannot vote in polls in this forum
You cannot attach files in this forum
You can download files in this forum
|
Powered by phpBB © 2001, 2005 phpBB Group
|



